The Germany Growth Hormone Deficiency Clinical Trial Market size was valued at USD 72 million in 2023. The total Germany Growth Hormone Deficiency Clinical Trial Market revenue is expected to grow at a CAGR of 4.2% from 2023 to 2030, reaching nearly USD 96.03 Million.Germany Growth Hormone Deficiency Clinical Trial Market Analysis
The MMR Comprehensive research report aims to provide a detailed analysis of key aspects of influencing the landscape. Germany is currently hosting Phase III Growth Hormone Deficiency (GHD) clinical trials, exploring diverse therapies, including traditional GH, LA-GH, gene therapy, and combination treatments. The average revenue per trial, which varies according to trial phase and therapy type, is estimated at XX million. It estimates a significant revenue potential of XX Million per year from the ongoing GHD clinical trials in Germany. Investors are interested in early-stage firms developing breakthrough growth hormone deficiency (GHD) Medicines, Digital Health Systems for Patient Management, And Specialist Contract research organizations (CROs) focused on GHD Trials. Profit margins in the German growth Hormone Deficiency (GHD) clinical trial business, which reflects the wider clinical trial landscape, are typically between 25% for both pharmaceutical companies and Contract Research Organizations (CROs). Several factors affect these margins, including the trial phase. Phase III trials are more profitable thanks to larger patient populations and clearer medication efficacy data.
Investment in GHD Clinical Trails and their Impact in Germany 1. Investments in patient-centered research approaches are expected to improve patient satisfaction by 12% compared to traditional trials. 2. According to MMR research, German GHD research is expected to secure an additional 65 million in funding by 2030, accelerating progress and attracting global recognition. To know about the Research Methodology :- Request Free Sample Report Patient Centric Approch In GHD Clinical Trails Patients actively participate in the design of modern clinical trials, providing insights into procedures and prioritizing outcomes that are important to them. Their engagement includes offering feedback on treatment design and ensuring research corresponds with patient interests. The patient-centered approach, which goes beyond typical clinical objectives, promotes patient-reported outcomes (PROs). These indicators examine the treatment's influence on daily living in a comprehensive manner, taking into account factors such as fatigue, emotional well-being, and treatment burden. PROs improve understanding of therapy effectiveness from the patient's perspective, allowing for a more comprehensive review. Impact on GHD Clinical Trails 1. Patient-centered studies are more encouraging to participants, resulting in more rapid recruitment and lower dropout rates. It saves time and resources, hence increasing the impact of research. 2. PROs and patient comments generate more data, providing for a more complete assessment of therapy efficacy and adverse effects. It also results in more robust evidence and dependable judgments.
Technological Integration in GHD Implementing and integrating advanced technologies in clinical trials, such as electronic data capture and remote monitoring face challenges related to standardization and compatibility. Standardization and compatibility are major problems in the German Growth Hormone Deficiency Clinical Trial Market. Various technical platforms frequently lack compatibility, requiring complex integration efforts and resulting in data silos. It hampers the easy exchange and analysis of data across several research sites. In addition, patient data privacy and security are top priorities. To overcome these problems, strong data protection policies and compliance with legislation are required. Also, the digital divide and accessibility difficulties result in differences in technology access across regions and demographics. The disparity is expected to exclude certain patient populations from clinical trials, limiting their participation and potentially twisting research results.

Germany Growth Hormone Deficiency Clinical Trial Market Segment Analysis
By Distribution Channel, Hospitals and pharmacies segments hold the dominant share of the German growth Hormone Deficiency Clinical Trial market, estimated at around 62%. Hospital pharmacies dominate Germany's Growth Hormone Deficiency Clinical Trial Market because of strict rules. GHD treatment requires specialized handling and monitoring, which balances the experience and equipment of hospital pharmacists. Direct patient access, as GHD patients frequently receive treatment in hospitals, adds to the ease of completing clinical trials via these pharmacies.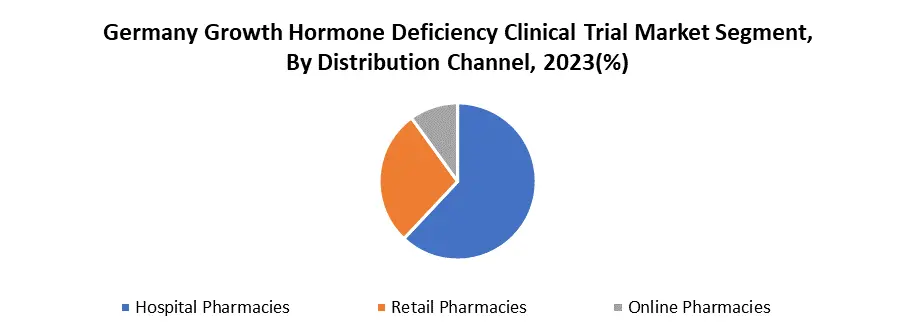
Germany Growth Hormone Deficiency Clinical Trials Market Scope: Inquire Before Buying
Germany Growth Hormone Deficiency Clinical Trials Market Report Coverage Details Base Year: 2023 Forecast Period: 2024-2030 Historical Data: 2018 to 2023 Market Size in 2023: US $ 72 Mn. Forecast Period 2024 to 2030 CAGR: 4.2% Market Size in 2030: US $ 96.02 Mn. Segments Covered: by Product Powder Solvent by Application Growth Hormone Deficiency Idiopathic Short Stature Turner Syndrome Small For Gestational Age Prader-Willi Syndrome by Distribution Channel Hospital Pharmacies Retail Pharmacies Online Pharmacies Key Players in the Germany Growth Hormone Deficiency Clinical Trial Market
1. Eli Lilly and Company 2. AnkeBio Co., Ltd 3. GeneScience Pharmaceuticals Co., Ltd 4. Novartis AG FAQs: 1. What are the different types of GHD clinical trials conducted in Germany? Ans. Phase I to IV trials exploring various aspects of GHD treatment, including safety, efficacy, and dosage optimization, are conducted. 2. How are GHD clinical trials regulated in Germany? Ans. The Federal Institute for Drugs and Medical Devices (BfArM) sets strict guidelines for ethical conduct, patient safety, and data privacy. 3. What is the projected market size & and growth rate of the Germany Growth Hormone Deficiency Clinical Trial Market? Ans. The Germany Growth Hormone Deficiency Clinical Trial Market size was valued at USD 72 Million in 2023. The total Germany Growth Hormone Deficiency Clinical Trial market revenue is expected to grow at a CAGR of 4.2% from 2023 to 2030, reaching nearly USD 96.02 Million By 2030.
1. Germany Growth Hormone Deficiency Clinical Trial Market Introduction 1.1. Study Assumption and Market Definition 1.2. Scope of the Study 1.3. Executive Summary 2. Germany Growth Hormone Deficiency Clinical Trial Market: Dynamics 2.1. Germany Growth Hormone Deficiency Clinical Trial Market Trends 2.2. PORTER’s Five Forces Analysis 2.3. PESTLE Analysis 2.4. Value Chain Analysis 2.5. Regulatory Landscape of Germany Growth Hormone Deficiency Clinical Trial Market 2.6. Technological Advancements in Australian Growth Hormone Deficiency Clinical Trial Market 2.7. Factors Driving the Growth of the Growth Hormone Deficiency Clinical Trial Market in Australia 2.8. Implication for the Healthcare Industry 2.9. Number of ongoing Trials 2.10. Phases of Clinical Trials in Germany 2.11. The Increased Focus on Patients Centricity 2.12. The Power of Technology in Clinical Trails 2.13. Key Opinion Leader Analysis for the Australian Growth Hormone Deficiency Clinical Trial Industry 2.14. Germany Growth Hormone Deficiency Clinical Trial Market Price Trend Analysis (2022-23) 3. Germany Growth Hormone Deficiency Clinical Trial Market: Market Size and Forecast by Segmentation for (by Value in USD Million) (2023-2030) 3.1. Germany Growth Hormone Deficiency Clinical Trial Market Size and Forecast, by Product (2023-2030) 3.1.1. Powder 3.1.2. Solvent 3.2. Germany Growth Hormone Deficiency Clinical Trial Market Size and Forecast, by Application (2023-2030) 3.2.1. Growth Hormone Deficiency 3.2.2. Idiopathic Short Stature 3.2.3. Turner Syndrome 3.2.4. Small For Gestational Age 3.2.5. Prader-Willi Syndrome 3.2.6. Germany Growth Hormone Deficiency Clinical Trial Market Size and Forecast, by Distribution Channel (2023-2030) 3.2.7. Hospital Pharmacies 3.2.8. Retail Pharmacies 3.2.9. Online Pharmacies 4. Germany Growth Hormone Deficiency Clinical Trial Market: Competitive Landscape 4.1. MMR Competition Matrix 4.2. Competitive Landscape 4.3. Key Players Benchmarking 4.3.1. Company Name 4.3.2. Product Segment 4.3.3. End-user Segment 4.3.4. Revenue (2023) 4.4. Market Analysis by Organized Players vs. Unorganized Players 4.4.1. Organized Players 4.4.2. Unorganized Players 4.5. Leading Germany Growth Hormone Deficiency Clinical Trial Market Companies, by market capitalization 4.6. Market Trends and Challenges in Australia 4.6.1. Technological Advancements 4.6.2. Affordability and Accessibility 4.6.3. Shortage of Skilled Professionals 4.7. Market Structure 4.7.1. Market Leaders 4.7.2. Market Followers 4.7.3. Emerging Players in the Market 4.7.4. Challenges 4.7.5. Mergers and Acquisitions Details 5. Company Profile: Key Players 5.1. Eli Lilly and Company 5.1.1. Company Overview 5.1.2. Business Portfolio 5.1.3. Financial Overview 5.1.4. SWOT Analysis 5.1.5. Strategic Analysis 5.1.6. Details on Partnership 5.1.7. Potential Impact of Emerging Technologies 5.1.8. Regulatory Accreditations and Certifications Received by Them 5.1.9. Strategies Adopted by Key Players 5.1.10. Recent Developments 5.2. AnkeBio Co., Ltd 5.3. GeneScience Pharmaceuticals Co., Ltd 5.4. Novartis AG 6. Key Findings 7. Industry Recommendations 8. Germany Growth Hormone Deficiency Clinical Trial Market: Research Methodology