The Clinical Trials Market size was valued at USD 58.19 Billion in 2025 and the total Clinical Trials revenue is expected to grow at a CAGR of 6.13% from 2025 to 2032, reaching nearly USD 88.25 Billion by 2032. Clinical trials are a type of research that studies new tests and treatments and evaluates their effects on human health outcomes. The Clinical trials market covers all the testing procedures conducted on a product before its approval in the market. With a focus on developing innovative therapies and treatments, the Clinical trials market has expected substantial growth globally, driven by key players such as IQVIA, PPD, Parexel International, and Charles River Laboratories. These companies have shaped the landscape through their strategic initiatives, collaborations, and technological integrations. Recent developments showcase a shift toward decentralized and virtual trials, leveraging digital tools and telemedicine to enhance patient recruitment, data collection, and monitoring, thereby improving efficiency and reducing costs. The COVID-19 pandemic accelerated the adoption of remote trial methodologies, fostering a greater acceptance of decentralized approaches. Regulatory advancements, such as the FDA's acceptance of real-world evidence, have also played a crucial role in transforming the Clinical trials market dynamics. Factors such as the demand for personalized medicine, increasing biopharmaceutical R&D expenditure, and the need for more efficient trial processes are expected to sustain the market's growth trajectory. Additionally, the emphasis on patient-centric approaches, coupled with investments in AI and data analytics, is poised to further revolutionize the clinical trial landscape, making trials more accessible, streamlined, and effective in bringing novel therapies to the Clinical trials market.To know about the Research Methodology :- Request Free Sample Report
Clinical Trials Market Dynamics:
Growing Demand for Personalized Therapies and Real-World Evidence driving the market growth The increasing number of clinical trials globally is driving the growth of the clinical trial market forward, supported by a heightened focus on patient safety, necessitating an increased requirement for these trials. This surge is further intensified by stringent approval mandates, compelling companies to conduct multiple trials to gain product endorsement. Notably, Europe and North America, particularly the United States, have emerged as pivotal regions, hosting a significant share of these trials, with the US registering over 30% by January 2023. The decentralization of clinical trials has witnessed a remarkable 25% increase in 2023, notably boosting the market's momentum. The clinical trial market is boosted by the alarming prevalence of chronic disorders globally, accounting for 74% of deaths, with approximately 125 million citizens in the US afflicted with chronic conditions. This staggering disease burden, coupled with a growth in infectious diseases, has fueled a substantial demand for new drugs, thereby intensifying the need for swift and effective clinical trials, consequently driving clinical trials market growth. A substantial focus on cancer therapy emerges as a significant driver, with a majority of new molecules dedicated to combating cancer. The pharmaceutical industry's attention towards cancer, given its status as a leading cause of global mortality, necessitates detailed clinical trials for new cancer drugs, expected to positively impact the clinical trials market during the forecast period. In addition to these drivers, technological integration with AI and machine learning expedites trial processes, while the adoption of decentralized trial models, acceptance of real-world evidence by regulatory bodies, and the growing demand for personalized therapies further fuel the clinical trials market. Collaborations between academia, industry, and patient advocacy groups, coupled with expansion into emerging markets and therapeutic diversification, all contribute to accelerating research and trial progress, shaping the upward trajectory of the clinical trial market.High Costs and Regulations Hamper Market Growth in Clinical Trials Stringent regulatory standards hinder the growth of the clinical trials market, for example, AstraZeneca's COVID-19 vaccine approval delays due to FDA standards, extended timelines and amplify costs. Recruitment struggles, seen in Pfizer's COVID-19 vaccine trial, impede progress. The capital-intensive nature of trials, showcased by Moderna's substantial COVID-19 vaccine trial expenditure, underscores financial burdens. Integrating new technologies, such as IBM's blockchain utilization in trials, demands substantial investments and infrastructure alterations. Unforeseen events like global pandemics, such as COVID-19, disrupt trials worldwide, impacting timelines and protocols. Diverse government regulations, highlighted by variations in drug approval criteria set by different agencies like the European Medicines Agency, add complexity. Ethical dilemmas, evidenced by controversies surrounding Pfizer's clinical trial in Nigeria, affect trial credibility. Data security breaches, like the University of California's incident, underscore vulnerabilities in patient information protection. Complex trial designs faced by Johnson & Johnson in HIV vaccine trials pose implementation challenges. Market adoption delays, exemplified by oncology drug acceptance post-trial, hinder industry growth by impeding the translation of successful trials into Clinical Trials Market acceptance.
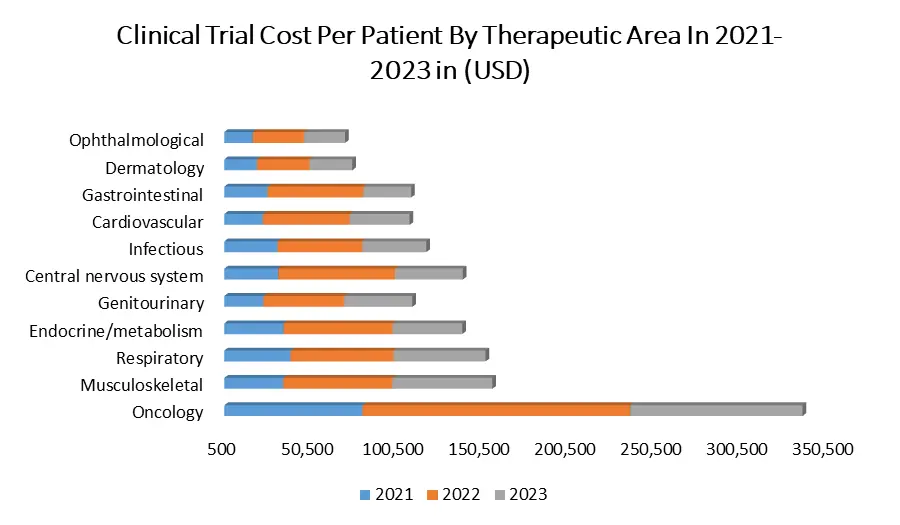
Clinical Trials Market Segment Analysis:
Based on Phase, In the clinical trial market, Phase III trials stand out due to their pivotal role in evaluating new medications against existing ones and confirming safety and effectiveness. These trials involve sizable participant groups, often up to 3,000 individuals, and span several years, making them complex endeavors. Phase III trials outnumber Phase I and Phase II trials due to their intricacy and demand for larger patient cohorts. The segment's growth is attributed to factors like burgeoning research activities, increasing disease burdens, and a plethora of investigative drugs in Phase III. The market segment's expansion is evident through the substantial volume of Phase III trials, as observed in clinicaltrials.gov, featuring 9,137 trials for cancer, 5,069 for cardiology, and 5,217 for respiratory studies, signifying significant contributions to this segment's growth. The Clinical trial market players' active involvement in Phase III trials, such as Lipidor AB's study on AKP02 skin spray for psoriasis and Wockhardt Ltd's global study on the new antibiotic WCK 5222, showcases the segment's vibrancy and its contribution to the market's upward trajectory. Based on the service type, Laboratory services dominated the clinical trials market in 2025 registering the highest growth within the market. This service type finds application across all phases of clinical trials, playing a pivotal role in determining the quality and efficacy of products through diverse qualitative and quantitative testing methodologies conducted within laboratories. Clinical trial supply and logistic services are poised for substantial growth in the forecast period due to their critical role in managing the supply chain for volunteers and logistical operations throughout clinical trials. The rise in the number of clinical trials globally acts as a primary driver for the increased demand for these services, emphasizing their significance in facilitating smooth trial operations. As a result, while laboratory services continue to uphold their prominence throughout all trial phases, clinical trial logistic and supply services are anticipated to witness significant adoption and application, marking an essential area of growth within the clinical trial market landscape.Clinical Trials Market Regional Insights:
North America dominated the clinical trials market and is expected to continue its dominance during the forecast period. The United States, a key contributor, faces a substantial burden of cancer, with an estimated 1,918,030 new cases projected in 2025, elevating the demand for innovative drugs and devices to diagnose and treat this ailment, thereby fueling clinical trials market expansion. Governmental support further augments growth prospects. For instance, Canada's launch of the Clinical Trials Fund (CTF), backed by a USD 250 million investment over three years, aims to fortify health outcomes, enhance clinical trials infrastructure, and nurture new clinical researchers, bolstering the region's clinical trial landscape. The major market players such as Janssen's initiation of Phase III trials for an investigational respiratory syncytial virus (RSV) vaccine among older adults in North America and other regions exemplify the region's innovation prowess, poised to drive market growth. Such initiatives and continuous advancements are anticipated to propel the North American clinical trials market forward.Clinical Trials Industry Ecosystem:
Clinical Trials Market Competitive Landscapes: The clinical trial market is highly competitive with several companies standing out across different tiers, each showcasing unique approaches and recent developments. Leading firms such as IQVIA drive innovation with decentralized trials, employing AI and analytics for enhanced efficiency. PPD's adaptive trial designs and real-time analytics expedite decision-making, while Parexel International emphasizes patient-centricity through remote monitoring tools. Following companies such as Medable focus on decentralized platforms, reducing patient dropout rates, ICON pioneers synthetic control arms, and Covance leads in biomarker-driven trials. Emerging players like Deep 6 AI swiftly identify eligible patients, Science 37 revolutionizes virtual trial methodologies, and TriNetX offers real-time data platforms for optimized trial design. Recent developments include IQVIA's healthcare system partnerships, PPD's virtual trial technologies, and Parexel's integration of wearables. Medable's success in patient retention, ICON's adoption of innovative trial designs, and Covance's advancements in biomarker-driven approaches highlight their contributions. These companies collectively propel the clinical trial landscape, blending technology, patient-centricity, and innovative methodologies to revolutionize therapeutic research and drive industry advancements.
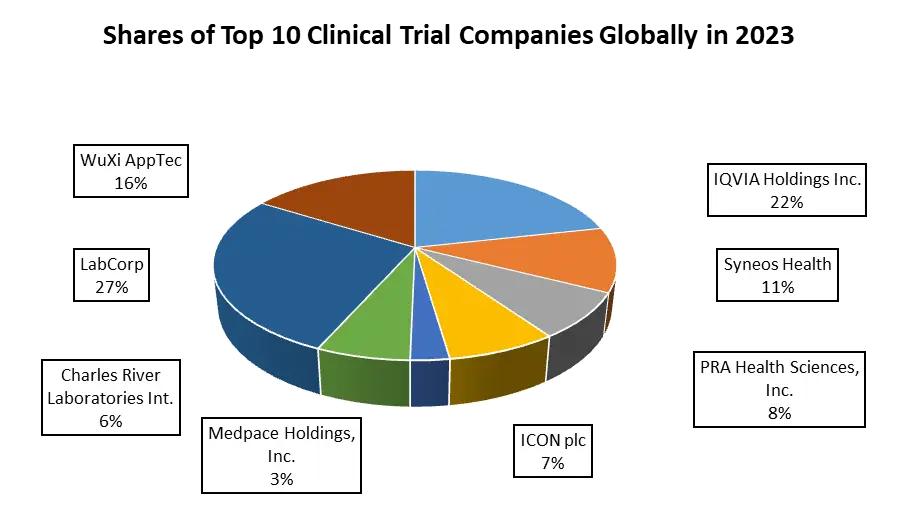
Clinical Trials Market Scope: Inquire before buying
Clinical Trials Market Report Coverage Details Base Year: 2025 Forecast Period: 2026-2032 Historical Data: 2020 to 2025 Market Size in 2025: USD 58.19 Bn. Forecast Period 2026 to 2032 CAGR: 6.13% Market Size in 2032: USD 88.25 Bn. Segments Covered: by Phase Phase I Phase II Phase III Phase IV by Service Type Protocol Designing Site Identification Patient Recruitment Laboratory Services Bioanalytical Testing Services Analytical Testing Services Clinical Trials Supply & Logistic Services Decentralized Clinical Services Clinical Trial Management Services Medical Device Testing Services Other clinical trial services by Indication Oncology Infectious Diseases Cardiology Neurology Women’s Health Genetic Diseases Immunology Other Therapy Area’s by Study Design Interventional Trials Observational Trials Expanded Access Trials Clinical Trials Market, by Region
North America (United States, Canada and Mexico) Europe (UK, France, Germany, Italy, Spain, Sweden, Austria and Rest of Europe) Asia Pacific (China, South Korea, Japan, India, Australia, Indonesia, Malaysia, Vietnam, Taiwan, Bangladesh, Pakistan and Rest of APAC) Middle East and Africa (South Africa, GCC, Egypt, Nigeria and Rest of ME&A) South America (Brazil, Argentina Rest of South America)Clinical Trials Market, Key Players:
Leading players of clinical trial in North America: 1. IQVIA Holdings Inc. 2. PPD 3. Syneos Health 4. PRA Health Sciences, Inc. 5. LabCorp (Laboratory Corporation of America Holdings) Major Clinical Trials providers in Europe: 1. Parexel International Corporation 2. ICON plc 3. Medpace Holdings, Inc. 4. Charles River Laboratories International, Inc. 5. Covance Inc. (part of LabCorp) Major Clinical Trials Market players Asia-Pacific: 1. WuXi AppTec 2. Novotech 3. CMIC Holdings Co., Ltd. 4. EPS International Co., Ltd. 5. Catalyst Clinical Services Pvt. Ltd. South America Clinical Trials Market Key Players: 1. Medpace Holdings, Inc. 2. PPD 3. ICON plc 4. PRA Health Sciences, Inc. 5. LabCorp (Laboratory Corporation of America Holdings) Middle East & Africa: 1. ICON plc 2. PRA Health Sciences, Inc. 3. IQVIA Holdings Inc. 4. Parexel International Corporation 5. PPD FAQs: 1. What are the growth drivers for the market? Ans. The Increased security concerns across diverse sectors such as Decentralized Trials, Precision Medicine Demand, and Patient-Centric Approaches are expected to be the major driver for the market. 2. What is the major restraint for the market growth? Ans. Stringent government regulations are expected to be the major restraining factor for the Clinical Trials market growth. 3. Which region is expected to lead the global Clinical Trials market during the forecast period? Ans. North America is expected to lead the global Clinical Trials market during the forecast period. 4. What is the projected market size and growth rate of the Clinical Trials Market? Ans. The Clinical Trials Market size was valued at USD 58.19 Billion in 2025 and the total Clinical Trials revenue is expected to grow at a CAGR of 6.13% from 2025 to 2032, reaching nearly USD 88.25 Billion by 2032. 5. What segments are covered in the Clinical Trials Market report? Ans. The segments covered in the Clinical Trials market report are Phase, Service Type, Indication, Study Design and Region.
1. Clinical Trials Market Introduction 1.1. Study Assumption and Market Definition 1.2. Scope of the Study 1.3. Executive Summary 2. Global Clinical Trials Market: Competitive Landscape 2.1. MMR Competition Matrix 2.2. Competitive Landscape 2.3. Market share analysis of major players 2.4. Key Players Benchmarking 2.4.1. Company Name 2.4.2. Product Segment 2.4.3. End-user Segment 2.4.4. Revenue (2023) 2.4.5. Geographic distribution of major customers 2.5. Market Structure 2.5.1. Market Leaders 2.5.2. Market Followers 2.5.3. Emerging Players 2.6. Mergers and Acquisitions Details 3. Market Size Estimation Methodology 3.1.1. Bottom-Up Approach 3.1.2. Top-Down Approach 4. Market Dynamics 4.1. Clinical Trials Market Trends By Region 4.1.1. North America Clinical Trials Market Trends 4.1.2. Europe Clinical Trials Market Trends 4.1.3. Asia Pacific Clinical Trials Market Trends 4.1.4. South America Clinical Trials Market Trends 4.1.5. Middle East & Africa (MEA) Clinical Trials Market Trends 4.2. Clinical Trials Market Dynamics 4.2.1. Drivers 4.2.2. Restraints 4.2.3. Opportunities 4.2.4. Challenges 4.3. PORTER’s Five Forces Analysis 4.3.1. Threat of New Entrants 4.3.2. Threat of Substitutes 4.3.3. Bargaining Power of Suppliers 4.3.4. Bargaining Power of Buyers 4.3.5. Intensity of Competitive Rivalry 4.4. PESTLE Analysis 4.5. Regulatory Landscape By Region 4.5.1. North America 4.5.2. Europe 4.5.3. Asia Pacific 4.5.4. South America 4.5.5. MEA 4.6. Analysis of Government Schemes and Initiatives For the Clinical Trials Industry 4.7. Pricing Analysis 4.8. Clinical Trials Industry Ecosystem 4.8.1. Key Players in the Clinical Trials Industry Ecosystem 4.8.2. Role of Companies in the Clinical Trials Industry Ecosystem 4.9. Technology Analysis 4.10. Investment and Funding Scenario 4.10.1. Major Investment and Funding 5. Clinical Trials Market: Global Market Size and Forecast By Segmentation (By Value USD Bn) (2025-2032) 5.1. Clinical Trials Market Size and Forecast, By Phase(2025-2032) 5.1.1. Phase I 5.1.2. Phase II 5.1.3. Phase III 5.1.4. Phase IV 5.2. Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 5.2.1. Protocol Designing 5.2.2. Site Identification 5.2.3. Patient Recruitment 5.2.4. Laboratory Services 5.2.5. Bioanalytical Testing Services 5.2.6. Analytical Testing Services 5.2.7. Clinical Trials Supply & Logistic Services 5.2.8. Decentralized Clinical Services 5.2.9. Clinical Trial Management Services 5.2.10. Medical Device Testing Services 5.2.11. Other clinical trial services 5.3. Clinical Trials Market Size and Forecast, By Indication(2025-2032) 5.3.1. Oncology 5.3.2. Infectious Diseases 5.3.3. Cardiology 5.3.4. Neurology 5.3.5. Women’s Health 5.3.6. Genetic Diseases 5.3.7. Immunology 5.3.8. Other Therapy Areas 5.4. Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 5.4.1. Interventional Trials 5.4.2. Observational Trials 5.4.3. Expanded Access Trials 5.5. Clinical Trials Market Size and Forecast, By Region (2025-2032) 5.5.1. North America 5.5.2. Europe 5.5.3. Asia Pacific 5.5.4. South America 5.5.5. MEA 6. North America Clinical Trials Market Size and Forecast By Segmentation (By Value USD Bn) (2025-2032) 6.1. North America Clinical Trials Market Size and Forecast, By Phase(2025-2032) 6.1.1. Phase I 6.1.2. Phase II 6.1.3. Phase III 6.1.4. Phase IV 6.2. North America Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 6.2.1. Protocol Designing 6.2.2. Site Identification 6.2.3. Patient Recruitment 6.2.4. Laboratory Services 6.2.5. Bioanalytical Testing Services 6.2.6. Analytical Testing Services 6.2.7. Clinical Trials Supply & Logistic Services 6.2.8. Decentralized Clinical Services 6.2.9. Clinical Trial Management Services 6.2.10. Medical Device Testing Services 6.2.11. Other clinical trial services 6.3. North America Clinical Trials Market Size and Forecast, By Indication(2025-2032) 6.3.1. Oncology 6.3.2. Infectious Diseases 6.3.3. Cardiology 6.3.4. Neurology 6.3.5. Women’s Health 6.3.6. Genetic Diseases 6.3.7. Immunology 6.3.8. Other Therapy Area’s 6.4. North America Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 6.4.1. Interventional Trials 6.4.2. Observational Trials 6.4.3. Expanded Access Trials 6.5. North America Clinical Trials Market Size and Forecast, By Country (2025-2032) 6.5.1. United States 6.5.1.1. United States Clinical Trials Market Size and Forecast, By Phase(2025-2032) 6.5.1.1.1. Phase I 6.5.1.1.2. Phase II 6.5.1.1.3. Phase III 6.5.1.1.4. Phase IV 6.5.1.2. United States Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 6.5.1.2.1. Protocol Designing 6.5.1.2.2. Site Identification 6.5.1.2.3. Patient Recruitment 6.5.1.2.4. Laboratory Services 6.5.1.2.5. Bioanalytical Testing Services 6.5.1.2.6. Analytical Testing Services 6.5.1.2.7. Clinical Trials Supply & Logistic Services 6.5.1.2.8. Decentralized Clinical Services 6.5.1.2.9. Clinical Trial Management Services 6.5.1.2.10. Medical Device Testing Services 6.5.1.2.11. Other clinical trial services 6.5.1.3. United States Clinical Trials Market Size and Forecast, By Indication(2025-2032) 6.5.1.3.1. Oncology 6.5.1.3.2. Infectious Diseases 6.5.1.3.3. Cardiology 6.5.1.3.4. Neurology 6.5.1.3.5. Women’s Health 6.5.1.3.6. Genetic Diseases 6.5.1.3.7. Immunology 6.5.1.3.8. Other Therapy Area’s 6.5.1.4. United States Clinical Trials Market Size and Forecast By Study Design(2025-2032) 6.5.1.4.1. Interventional Trials 6.5.1.4.2. Observational Trials 6.5.1.4.3. Expanded Access Trials 6.5.2. Canada 6.5.2.1. Canada Clinical Trials Market Size and Forecast, By Phase(2025-2032) 6.5.2.1.1. Phase I 6.5.2.1.2. Phase II 6.5.2.1.3. Phase III 6.5.2.1.4. Phase IV 6.5.2.2. Canada Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 6.5.2.2.1. Protocol Designing 6.5.2.2.2. Site Identification 6.5.2.2.3. Patient Recruitment 6.5.2.2.4. Laboratory Services 6.5.2.2.5. Bioanalytical Testing Services 6.5.2.2.6. Analytical Testing Services 6.5.2.2.7. Clinical Trials Supply & Logistic Services 6.5.2.2.8. Decentralized Clinical Services 6.5.2.2.9. Clinical Trial Management Services 6.5.2.2.10. Medical Device Testing Services 6.5.2.2.11. Other clinical trial services 6.5.2.3. Canada Clinical Trials Market Size and Forecast, By Indication(2025-2032) 6.5.2.3.1. Oncology 6.5.2.3.2. Infectious Diseases 6.5.2.3.3. Cardiology 6.5.2.3.4. Neurology 6.5.2.3.5. Women’s Health 6.5.2.3.6. Genetic Diseases 6.5.2.3.7. Immunology 6.5.2.3.8. Other Therapy Area’s 6.5.2.4. Canada Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 6.5.2.4.1. Interventional Trials 6.5.2.4.2. Observational Trials 6.5.2.4.3. Expanded Access Trials 6.5.3. Mexico 6.5.3.1. Mexico Clinical Trials Market Size and Forecast, By Phase(2025-2032) 6.5.3.1.1. Phase I 6.5.3.1.2. Phase II 6.5.3.1.3. Phase III 6.5.3.1.4. Phase IV 6.5.3.2. Mexico Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 6.5.3.2.1. Protocol Designing 6.5.3.2.2. Site Identification 6.5.3.2.3. Patient Recruitment 6.5.3.2.4. Laboratory Services 6.5.3.2.5. Bioanalytical Testing Services 6.5.3.2.6. Analytical Testing Services 6.5.3.2.7. Clinical Trials Supply & Logistic Services 6.5.3.2.8. Decentralized Clinical Services 6.5.3.2.9. Clinical Trial Management Services 6.5.3.2.10. Medical Device Testing Services 6.5.3.2.11. Other clinical trial services 6.5.3.3. Mexico Clinical Trials Market Size and Forecast, By Indication(2025-2032) 6.5.3.3.1. Oncology 6.5.3.3.2. Infectious Diseases 6.5.3.3.3. Cardiology 6.5.3.3.4. Neurology 6.5.3.3.5. Women’s Health 6.5.3.3.6. Genetic Diseases 6.5.3.3.7. Immunology 6.5.3.3.8. Other Therapy Area’s 6.5.3.4. Mexico Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 6.5.3.4.1. Interventional Trials 6.5.3.4.2. Observational Trials 6.5.3.4.3. Expanded Access Trials 7. Europe Clinical Trials Market Size and Forecast By Segmentation (By Value USD Bn) (2025-2032) 7.1. Europe Clinical Trials Market Size and Forecast, By Phase(2025-2032) 7.2. Europe Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 7.3. Europe Clinical Trials Market Size and Forecast, By Indication(2025-2032) 7.4. Europe Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 7.5. Europe Clinical Trials Market Size and Forecast, By Country (2025-2032) 7.5.1. United Kingdom 7.5.1.1. United Kingdom Clinical Trials Market Size and Forecast, By Phase(2025-2032) 7.5.1.2. United Kingdom Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 7.5.1.3. United Kingdom Clinical Trials Market Size and Forecast, By Indication(2025-2032) 7.5.1.4. United Kingdom Clinical Trials Market Size and Forecast By Study Design(2025-2032) 7.5.2. France 7.5.2.1. France Clinical Trials Market Size and Forecast, By Phase(2025-2032) 7.5.2.2. France Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 7.5.2.3. France Clinical Trials Market Size and Forecast, By Indication(2025-2032) 7.5.2.4. France Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 7.5.3. Germany 7.5.3.1. Germany Clinical Trials Market Size and Forecast, By Phase(2025-2032) 7.5.3.2. Germany Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 7.5.3.3. Germany Clinical Trials Market Size and Forecast, By Indication(2025-2032) 7.5.3.4. Germany Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 7.5.4. Italy 7.5.4.1. Italy Clinical Trials Market Size and Forecast, By Phase(2025-2032) 7.5.4.2. Italy Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 7.5.4.3. Italy Clinical Trials Market Size and Forecast, By Indication(2025-2032) 7.5.4.4. Italy Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 7.5.5. Spain 7.5.5.1. Spain Clinical Trials Market Size and Forecast, By Phase(2025-2032) 7.5.5.2. Spain Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 7.5.5.3. Spain Clinical Trials Market Size and Forecast, By Indication(2025-2032) 7.5.5.4. Spain Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 7.5.6. Sweden 7.5.6.1. Sweden Clinical Trials Market Size and Forecast, By Phase(2025-2032) 7.5.6.2. Sweden Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 7.5.6.3. Sweden Clinical Trials Market Size and Forecast, By Indication(2025-2032) 7.5.6.4. Sweden Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 7.5.7. Austria 7.5.7.1. Austria Clinical Trials Market Size and Forecast, By Phase(2025-2032) 7.5.7.2. Austria Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 7.5.7.3. Austria Clinical Trials Market Size and Forecast, By Indication(2025-2032) 7.5.7.4. Austria Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 7.5.8. Rest of Europe 7.5.8.1. Rest of Europe Clinical Trials Market Size and Forecast, By Phase(2025-2032) 7.5.8.2. Rest of Europe Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 7.5.8.3. Rest of Europe Clinical Trials Market Size and Forecast, By Indication(2025-2032) 7.5.8.4. Rest of Europe Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 8. Asia Pacific Clinical Trials Market Size and Forecast By Segmentation (By Value USD Bn) (2025-2032) 8.1. Asia Pacific Clinical Trials Market Size and Forecast, By Phase(2025-2032) 8.2. Asia Pacific Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 8.3. Asia Pacific Clinical Trials Market Size and Forecast, By Indication(2025-2032) 8.4. Asia Pacific Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 8.5. Asia Pacific Clinical Trials Market Size and Forecast, By Country (2025-2032) 8.5.1. China 8.5.1.1. China Clinical Trials Market Size and Forecast, By Phase(2025-2032) 8.5.1.2. China Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 8.5.1.3. China Clinical Trials Market Size and Forecast, By Indication(2025-2032) 8.5.1.4. China Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 8.5.2. S Korea 8.5.2.1. S Korea Clinical Trials Market Size and Forecast, By Phase(2025-2032) 8.5.2.2. S Korea Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 8.5.2.3. S Korea Clinical Trials Market Size and Forecast, By Indication(2025-2032) 8.5.2.4. S Korea Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 8.5.3. Japan 8.5.3.1. Japan Clinical Trials Market Size and Forecast, By Phase(2025-2032) 8.5.3.2. Japan Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 8.5.3.3. Japan Clinical Trials Market Size and Forecast, By Indication(2025-2032) 8.5.3.4. Japan Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 8.5.4. India 8.5.4.1. India Clinical Trials Market Size and Forecast, By Phase(2025-2032) 8.5.4.2. India Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 8.5.4.3. India Clinical Trials Market Size and Forecast, By Indication(2025-2032) 8.5.4.4. India Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 8.5.5. Australia 8.5.5.1. Australia Clinical Trials Market Size and Forecast, By Phase(2025-2032) 8.5.5.2. Australia Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 8.5.5.3. Australia Clinical Trials Market Size and Forecast, By Indication(2025-2032) 8.5.5.4. Australia Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 8.5.6. ASEAN 8.5.6.1. ASEAN Clinical Trials Market Size and Forecast, By Phase(2025-2032) 8.5.6.2. ASEAN Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 8.5.6.3. ASEAN Clinical Trials Market Size and Forecast, By Indication(2025-2032) 8.5.6.4. ASEAN Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 8.5.7. Rest of Asia Pacific 8.5.7.1. Rest of Asia Pacific Clinical Trials Market Size and Forecast, By Phase(2025-2032) 8.5.7.2. Rest of Asia Pacific Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 8.5.7.3. Rest of Asia Pacific Clinical Trials Market Size and Forecast, By Indication(2025-2032) 8.5.7.4. Rest of Asia Pacific Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 9. South America Clinical Trials Market Size and Forecast By Segmentation (By Value USD Bn) (2025-2032) 9.1. South America Clinical Trials Market Size and Forecast, By Phase(2025-2032) 9.2. South America Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 9.3. South America Clinical Trials Market Size and Forecast, By Indication(2025-2032) 9.4. South America Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 9.5. South America Clinical Trials Market Size and Forecast, By Country (2025-2032) 9.5.1. Brazil 9.5.1.1. Brazil Clinical Trials Market Size and Forecast, By Phase(2025-2032) 9.5.1.2. Brazil Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 9.5.1.3. Brazil Clinical Trials Market Size and Forecast, By Indication(2025-2032) 9.5.1.4. Brazil Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 9.5.2. Argentina 9.5.2.1. Argentina Clinical Trials Market Size and Forecast, By Phase(2025-2032) 9.5.2.2. Argentina Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 9.5.2.3. Argentina Clinical Trials Market Size and Forecast, By Indication(2025-2032) 9.5.2.4. Argentina Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 9.5.3. Rest Of South America 9.5.3.1. Rest Of South America Clinical Trials Market Size and Forecast, By Phase(2025-2032) 9.5.3.2. Rest Of South America Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 9.5.3.3. Rest Of South America Clinical Trials Market Size and Forecast, By Indication(2025-2032) 9.5.3.4. Rest Of South America Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 10. Middle East and Africa Clinical Trials Market Size and Forecast By Segmentation (By Value USD Bn) (2025-2032) 10.1. Middle East and Africa Clinical Trials Market Size and Forecast, By Phase(2025-2032) 10.2. Middle East and Africa Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 10.3. Middle East and Africa Clinical Trials Market Size and Forecast, By Indication(2025-2032) 10.4. Middle East and Africa Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 10.5. Middle East and Africa Clinical Trials Market Size and Forecast, By Country (2025-2032) 10.5.1. South Africa 10.5.1.1. South Africa Clinical Trials Market Size and Forecast, By Phase(2025-2032) 10.5.1.2. South Africa Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 10.5.1.3. South Africa Clinical Trials Market Size and Forecast, By Indication(2025-2032) 10.5.1.4. South Africa Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 10.5.2. GCC 10.5.2.1. GCC Clinical Trials Market Size and Forecast, By Phase(2025-2032) 10.5.2.2. GCC Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 10.5.2.3. GCC Clinical Trials Market Size and Forecast, By Indication(2025-2032) 10.5.2.4. GCC Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 10.5.3. Rest Of MEA 10.5.3.1. Rest Of MEA Clinical Trials Market Size and Forecast, By Phase(2025-2032) 10.5.3.2. Rest Of MEA Clinical Trials Market Size and Forecast, By Service Type(2025-2032) 10.5.3.3. Rest Of MEA Clinical Trials Market Size and Forecast, By Indication(2025-2032) 10.5.3.4. Rest Of MEA Clinical Trials Market Size and Forecast, By Study Design(2025-2032) 11. Company Profile: Key Players 11.1. IQVIA Holdings Inc. 11.1.1. Company Overview 11.1.2. Business Portfolio 11.1.3. Financial Overview 11.1.4. SWOT Analysis (Technological strengths and weaknesses) 11.1.5. Strategic Analysis (Recent strategic moves) 11.1.6. Recent Developments 11.2. PPD 11.3. Syneos Health 11.4. PRA Health Sciences, Inc. 11.5. LabCorp 11.6. Parexel International Corporation 11.7. ICON plc 11.8. Medpace Holdings, Inc. 11.9. Charles River Laboratories International, Inc. 11.10. WuXi AppTec 11.11. Novotech 11.12. CMIC Holdings Co., Ltd. 11.13. EPS International Co., Ltd. 11.14. Catalyst Clinical Services Pvt. Ltd. 11.15. PRA Health Sciences, Inc. 11.16. Worldwide Clinical Trails 11.17. KCR 11.18. Pharm-Olam International 11.19. Cinipaxe 11.20. Ergomed 12. Key Findings 13. Analyst Recommendations 13.1. Attractive Opportunities for Players in the Clinical Trials Market 13.2. Future Outlooks 14. Clinical Trials Market: Research Methodology
