Global Preclinical CRO Market size was valued at USD 7.19 Bn in 2024 and the total Global Preclinical CRO Market revenue is expected to grow at 8.06% through 2025 to 2032, reaching nearly USD 13.37 Bn.Global Preclinical CRO Market Introduction
Preclinical Contract Research Organization (CRO) is specialized service provider that partner with pharmaceutical, biotechnology and medical device company to conduct essential research and testing before human clinical trial. Global preclinical CRO market has been experiencing robust growth fueled by rising R&D investment, government prioritization of life saving therapy and regulatory reform like U.S. 21st Century Cures Act and evolving EU toxicity testing requirement. North America dominated Global preclinical CRO market, driven by its concentrated biopharma industry, FDA modernization initiative and strong public private partnership while Asia Pacific emerge as a high growth region due to cost advantage and increasing outsourcing. Preclinical CROs offer key benefit such as specialized expertise, regulatory compliance and scalable infrastructure making them indispensable for efficient drug development. Leading players like Charles River Laboratories, LabCorp and WuXi AppTec are advancing market through AI driven predictive toxicology, organ on chip technology and genetically engineered model to improve translational accuracy. With growing complexity of biologic therapies, orphan drug and personalized medicine, preclinical CROs are becoming vital partners in reducing development timelines positioning market for sustained expansion. Key demand stems from small molecule drug developers, large biopharma firms and academic research institution particularly in oncology, neurology and rare disease segments.To know about the Research Methodology :- Request Free Sample Report
Global Preclinical CRO Market Dynamics
Rising Demand for Preclinical CRO Services to Drive Global Preclinical CRO Market The need for preclinical contract research organization (CRO) services has grown in recent years as the R&D budget for drug development has expanded, supporting market growth over the forecast period. Preclinical research initiatives have been hampered by the ongoing Covid-19 epidemic, particularly in the first quarter of 2021. Nonetheless, life-saving medicines must be discovered and commercialized quickly. For many biopharmaceutical companies, preclinical CROs play a critical role in moving the concept to the commercialization stage. In the case of the COVID vaccine, preclinical investigations have been completed at an exponential rate, demonstrating CROs' dominant importance. Increasing expenditure on CRO services is projected to considerably enhance market growth during the forecast period. According to a survey conducted by Servier Research Institute in 2021, toxicological testing is responsible for about 50% of preclinical failure, which is expected to drive demand for preclinical CRO services in the future years. Legislative Reforms to Boost Global Preclinical CRO Market Aside from that, recent legislative changes in Europe pertaining to preclinical CRO services are expected to boost demand for toxicity testing, boosting the regional market growth. The procedure of medicine approved by the Food and Drug Administration has changed dramatically throughout the years (FDA). The United States recently enacted the 21st Century Cures Act, which streamlines the clearance process for breakthrough medicines and medical devices. Changes in approval processes are likely to spur innovation and raise demand for preclinical services, boosting the market growth. R&D Investments and Collaborative Models to Drive Global Preclinical CRO Market In addition, major drivers expected to fuel the growth of the worldwide preclinical CRO market include increased government attention on the development of life-saving medications, as well as growing demand for services such as bioanalysis and DMPK investigations, toxicity testing, and others. Since 2000, PhRMA member businesses have invested almost $1 trillion in R&D, making the biopharmaceutical industry the most R&D-intensive industry in the United States. Also, the growth of the preclinical CRO market is likely to be influenced by the increasing collaborative work approach among the public and government for the creation of innovative goods.Global Preclinical CRO Market Segment Analysis
Based on service, Global Preclinical CRO Market is segmented into toxicology testing, bioanalysis & DMPK Studies, etc. Due to an increase in the outsourcing of noncore preclinical CRO studies and strong uptake in toxicology tests, toxicology testing held the biggest revenue share of 61.1 % in 2024. The market for preclinical CRO is divided into three categories based on service: bioanalysis and DMPK research, toxicity testing, and others. Over the forecast period, the bioanalysis and DMPK studies segment is expected to grow at the fastest rate of xx%. A surge in demand for pharmacokinetic services to assist toxicological testing for IND-enabling studies is credited for this profitable growth. For thorough evaluation and analysis of effectiveness and safety data for new drug development, bioanalysis and DMPK investigations are also required. Furthermore, the results of non-clinical toxicological and pharmacological investigations must be compared to those of non-clinical and clinical pharmacokinetic research. Based on end-user, Global Preclinical CRO Market is segmented into biopharmaceutical companies, Government & Academic institutes and medical device companies. In 2024, the biopharmaceutical companies’ segment had the highest market share of 81.1 %. The rising trend of outsourcing end-to-end services among biopharmaceutical firms, particularly small and mid-size businesses that lack significant competence in the preclinical phase of drug development, is projected to increase demand for preclinical CRO services in the future. Over the forecast period, government and academic institutes are expected to increase the most. The market is divided into biopharmaceutical firms, government and academic institutes, and medical device businesses based on end-use.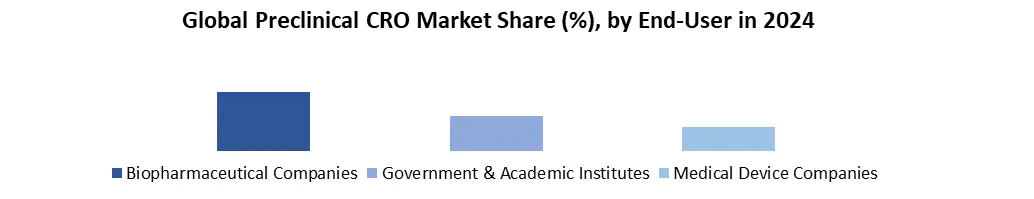
Global Preclinical CRO Market Regional Insights
North America Dominated Global Preclinical CRO Market With a market share of 47.7% in 2024. Due to increased R&D spending and increased adoption of new technologies, the area is anticipated to continue its dominance during the forecast period. Increased availability of trained human resources and decreased gadget costs are also expected to propel the market forward throughout the forecast period. According to a study published by ClinicalTrail.gov, the number of registered preclinical trials has grown dramatically in recent years. According to the study, there were 45,445 preclinical research registered in the United States alone in 2024. Furthermore, the regional industry demand is expected to be fuelled by pharmaceutical firms' increased focus on innovative medication development for the treatment of numerous chronic diseases. The rising prevalence of chronic illnesses is boosting demand for preclinical CRO services, which is driving the regional market growth. Due to the cost-effectiveness of preclinical CROs in countries like India and China, Asia Pacific is expected to grow at the fastest rate throughout the forecast period.Global Preclinical CRO Market Competitive Landscape WuXi AppTec dominates global preclinical CRO sector as China’s largest player competing with Western giants like Charles River Laboratories (US), LabCorp (US) and Eurofins (EU) through its end to end "CRDMO" (Contract Research, Development, and Manufacturing) model. Company differentiates itself with integrated services from discovery biology to IND enabling studies leveraging China’s cost advantages and scalable capacity. While rivals excel in niche area (e.g., Charles River in toxicology, LabCorp in bioanalysis) WuXi AppTec capture market share via aggressive expansion (e.g., $3B global R&D infrastructure investment in 2023) and AI driven platform for predictive toxicology. However, geopolitical tension (US BIOSECURE Act) and rising competition from regional peer challenge its Western market growth. WuXi AppTec’s Asia Pacific stronghold backed by China’s booming biopharma R&D fuels its lead in large molecule and cell/gene therapy preclinical service, positioning it as a critical partner for both global pharma and emerging biotechs. Global Preclinical CRO Market Key Trends • Increased adoption of AI and machine learning to enhance predictive toxicology and streamline preclinical study design in 2024. • Growing demand for human-relevant models (e.g., organoids, organ-on-a-chip) over traditional animal testing due to regulatory and ethical pressures. • Expansion of CROs in Asia-Pacific as pharmaceutical companies seek cost-efficient and high-quality preclinical research solutions. Global Preclinical CRO Market Key Developments • Covance Inc. (LabCorp)-US: March 2024: LabCorp’s Covance expanded its oncology preclinical services with new PDX (Patient-Derived Xenograft) models to enhance cancer drug discovery. • MD Biosciences, Inc – Canada: January 2025: MD Biosciences partnered with a Canadian biotech firm to develop AI-driven neuroinflammation models for neurodegenerative disease research. • Eurofins Scientific – Luxembourg: February 2024: Eurofins launched a new high-throughput ADME-Tox screening platform to accelerate early-stage drug safety assessments. • ICON Plc – Ireland: April 2024: ICON acquired a specialized preclinical imaging CRO to strengthen its translational research capabilities in metabolic diseases. • Jubilant Biosys – India: November 2024: Jubilant Biosys inaugurated a new preclinical research facility in Bangalore, focusing on AI-integrated drug discovery for infectious diseases.
Preclinical CRO Market Scope: Inquire before buying
Preclinical CRO Market Report Coverage Details Base Year: 2024 Forecast Period: 2025-2032 Historical Data: 2019 to 2024 Market Size in 2024: USD 7.19 Bn. Forecast Period 2025 to 2032 CAGR: 8.06% Market Size in 2032: USD 13.37 Bn. Segments Covered: by Service Bioanalysis and DMPK Studies Toxicology Testing Others by End Use Biopharmaceutical Companies Government and Academic Institutes Medical Device Companies Global Preclinical CRO Market, by region
North America (United States, Canada and Mexico) Europe (United Kingdom, France, Germany, Italy, Spain, Sweden, Russia, Rest of Europe) Asia Pacific (China, Japan, South Korea, India, Australia, Malaysia, Thailand, Vietnam, Indonesia, Philippines, Rest of APAC) Middle East and Africa (South Africa, GCC, Nigeria, Egypt, Turkey, Rest of MEA) South America (Brazil, Argentina, Colombia, Chile, Peru, Rest of South America)Global Preclinical CRO Market Key Players
North America 1. Envigo Corporation (US) 2. MPI Research (US) 3. PRA Health Sciences, Inc. (US) 4. Medpace, Inc. (US) 5. Pharmaceutical Product Development (PPD), LLC (US) 6. PARAXEL International Corporation (US) 7. Laboratory Corporation of America, Inc (US) 8. Covance Inc. (LabCorp) (US) 9. Charles River Laboratories (US) 10. Syneos Health (US) 11. Thermo Fisher Scientific (US) 12. Inotiv, Inc. (US) 13. MD Biosciences, Inc (Canada) Europe 14. Eurofins Scientific (Luxembourg) 15. ICON Plc (Ireland) 16. Admescope (Finland) Asia Pacific 17. Wuxi AppTec (China) 18. Crown Bioscience (China) 19. Jubilant Biosys (India) 20. Novotech (Australia)Frequently Asked Questions:
1. Which region has the largest share in Global Preclinical CRO Market? Ans: North America region held the highest share in 2024. 2. What is the growth rate of Global Preclinical CRO Market? Ans: The Global Preclinical CRO Market is growing at a CAGR of 8.06% during forecasting period 2025-2032. 3. What is scope of the Global Preclinical CRO market report? Ans: Global Preclinical CRO Market report helps with the PESTEL, PORTER, COVID-19 Impact analysis, Recommendations for Investors & Leaders, and market estimation of the forecast period. 4. Who are the key players in Global Preclinical CRO market? Ans: The important key players in the Global Preclinical CRO Market are – NeuroSky, EMOTIV, Advanced Brain Monitoring Inc., Koninklijke Philips N.V., Medtronic, Muse, Natus Medical Incorporated, Cadwell Industries Inc., NeuroWave Systems Inc., BrainScope., Hangzhou Zhongheng Electric, Deayea, NeuroTherapeutics, and Melon. 5. What is the study period of this market? Ans: The Global Preclinical CRO Market is studied from 2024 to 2032.
1. Global Preclinical CRO Market Introduction 1.1. Study Assumption and Market Definition 1.2. Scope of the Study 1.3. Executive Summary 2. Global Preclinical CRO Market: Competitive Landscape 2.1. Ecosystem Analysis 2.2. MMR Competition Matrix 2.3. Competitive Landscape 2.4. Key Players Benchmarking 2.4.1. Company Name 2.4.2. Business Segment 2.4.3. End-User Segment 2.4.4. Revenue (2024) 2.4.5. Company Locations 2.5. Market Structure 2.5.1. Market Leaders 2.5.2. Market Followers 2.5.3. Emerging Players 2.6. Mergers and Acquisitions Details 2.7. KANO Model Analysis 3. Global Preclinical CRO Market: Dynamics 3.1. Global Preclinical CRO Market Trends 3.1.1. North America Global Preclinical CRO Market Trends 3.1.2. Europe Global Preclinical CRO Market Trends 3.1.3. Asia Pacific Global Preclinical CRO Market Trends 3.1.4. Middle East and Africa Global Preclinical CRO Market Trends 3.1.5. South America Global Preclinical CRO Market Trends 3.2. Global Preclinical CRO Market Dynamics 3.2.1. Global Preclinical CRO Market Drivers 3.2.1.1. Rising Demand for Preclinical CRO Services 3.2.1.2. Legislative Reforms 3.2.1.3. R&D Investments 3.2.2. Global Preclinical CRO Market Restraints 3.2.3. Global Preclinical CRO Market Opportunities 3.2.4. Global Preclinical CRO Market Challenges 3.3. PORTER’s Five Forces Analysis 3.4. PESTLE Using Tree Map Analysis 3.4.1. Regulatory Shifts 3.4.2. Tech Advancements 3.4.3. Market Expansion 3.5. Regulatory Landscape by Region 3.5.1. North America 3.5.2. Europe 3.5.3. Asia Pacific 3.5.4. Middle East and Africa 3.5.5. South America 3.6. Key Opinion Leader Analysis for the Global Industry 3.7. Analysis of Government Schemes and Initiatives for Industry 4. Global Preclinical CRO Market: Global Market Size and Forecast by Segmentation (by Value in USD Bn) (2024-2032) 4.1. Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 4.1.1. Bioanalysis and DMPK Studies 4.1.2. Toxicology Testing 4.1.3. Others 4.2. Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 4.2.1. Biopharmaceutical Companies 4.2.2. Government and Academic Institutes 4.2.3. Medical Device Companies 4.3. Global Preclinical CRO Market Size and Forecast, By Region (2024-2032) 4.3.1. North America 4.3.2. Europe 4.3.3. Asia Pacific 4.3.4. Middle East and Africa 4.3.5. South America 5. North America Global Preclinical CRO Market Size and Forecast by Segmentation (by Value in USD Bn) (2024-2032) 5.1. North America Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 5.1.1. Bioanalysis and DMPK Studies 5.1.2. Toxicology Testing 5.1.3. Others 5.2. North America Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 5.2.1. Biopharmaceutical Companies 5.2.2. Government and Academic Institutes 5.2.3. Medical Device Companies 5.3. North America Global Preclinical CRO Market Size and Forecast, by Country (2024-2032) 5.3.1. United States 5.3.1.1. United States Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 5.3.1.1.1. Bioanalysis and DMPK Studies 5.3.1.1.2. Toxicology Testing 5.3.1.1.3. Others 5.3.1.2. United States Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 5.3.1.2.1. Biopharmaceutical Companies 5.3.1.2.2. Government and Academic Institutes 5.3.1.2.3. Medical Device Companies 5.3.2. Canada 5.3.2.1. Canada Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 5.3.2.1.1. Bioanalysis and DMPK Studies 5.3.2.1.2. Toxicology Testing 5.3.2.1.3. Others 5.3.2.2. Canada Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 5.3.2.2.1. Biopharmaceutical Companies 5.3.2.2.2. Government and Academic Institutes 5.3.2.2.3. Medical Device Companies 5.3.3. Mexico 5.3.3.1. Mexico Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 5.3.3.1.1. Bioanalysis and DMPK Studies 5.3.3.1.2. Toxicology Testing 5.3.3.1.3. Others 5.3.3.2. Mexico Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 5.3.3.2.1. Biopharmaceutical Companies 5.3.3.2.2. Government and Academic Institutes 5.3.3.2.3. Medical Device Companies 6. Europe Global Preclinical CRO Market Size and Forecast by Segmentation (by Value in USD Bn) (2024-2032) 6.1. Europe Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 6.2. Europe Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 6.3. Europe Global Preclinical CRO Market Size and Forecast, by Country (2024-2032) 6.3.1. United Kingdom 6.3.1.1. United Kingdom Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 6.3.1.2. United Kingdom Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 6.3.2. France 6.3.2.1. France Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 6.3.2.2. France Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 6.3.3. Germany 6.3.3.1. Germany Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 6.3.3.2. Germany Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 6.3.4. Italy 6.3.4.1. Italy Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 6.3.4.2. Italy Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 6.3.5. Spain 6.3.5.1. Spain Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 6.3.5.2. Spain Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 6.3.6. Sweden 6.3.6.1. Sweden Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 6.3.6.2. Sweden Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 6.3.7. Russia 6.3.7.1. Russia Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 6.3.7.2. Russia Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 6.3.8. Rest of Europe 6.3.8.1. Rest of Europe Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 6.3.8.2. Rest of Europe Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 7. Asia Pacific Global Preclinical CRO Market Size and Forecast by Segmentation (by Value in USD Bn) (2024-2032) 7.1. Asia Pacific Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 7.2. Asia Pacific Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 7.3. Asia Pacific Global Preclinical CRO Market Size and Forecast, by Country (2024-2032) 7.3.1. China 7.3.1.1. China Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 7.3.1.2. China Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 7.3.2. S Korea 7.3.2.1. S Korea Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 7.3.2.2. S Korea Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 7.3.3. Japan 7.3.3.1. Japan Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 7.3.3.2. Japan Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 7.3.4. India 7.3.4.1. India Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 7.3.4.2. India Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 7.3.5. Australia 7.3.5.1. Australia Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 7.3.5.2. Australia Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 7.3.6. Indonesia 7.3.6.1. Indonesia Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 7.3.6.2. Indonesia Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 7.3.7. Malaysia 7.3.7.1. Malaysia Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 7.3.7.2. Malaysia Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 7.3.8. Philippines 7.3.8.1. Philippines Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 7.3.8.2. Philippines Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 7.3.9. Thailand 7.3.9.1. Thailand Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 7.3.9.2. Thailand Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 7.3.10. Vietnam 7.3.10.1. Vietnam Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 7.3.10.2. Vietnam Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 7.3.11. Rest of Asia Pacific 7.3.11.1. Rest of Asia Pacific Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 7.3.11.2. Rest of Asia Pacific Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 8. Middle East and Africa Global Preclinical CRO Market Size and Forecast (by Value in USD Bn) (2024-2032) 8.1. Middle East and Africa Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 8.2. Middle East and Africa Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 8.3. Middle East and Africa Global Preclinical CRO Market Size and Forecast, by Country (2024-2032) 8.3.1. South Africa 8.3.1.1. South Africa Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 8.3.1.2. South Africa Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 8.3.2. GCC 8.3.2.1. GCC Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 8.3.2.2. GCC Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 8.3.3. Egypt 8.3.3.1. Egypt Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 8.3.3.2. Egypt Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 8.3.4. Nigeria 8.3.4.1. Nigeria Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 8.3.4.2. Nigeria Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 8.3.5. Rest of ME&A 8.3.5.1. Rest of ME&A Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 8.3.5.2. Rest of ME&A Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 9. South America Global Preclinical CRO Market Size and Forecast by Segmentation (by Value in USD Bn) (2024-2032) 9.1. South America Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 9.2. South America Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 9.3. South America Global Preclinical CRO Market Size and Forecast, by Country (2024-2032) 9.3.1. Brazil 9.3.1.1. Brazil Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 9.3.1.2. Brazil Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 9.3.2. Argentina 9.3.2.1. Argentina Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 9.3.2.2. Argentina Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 9.3.3. Colombia 9.3.3.1. Colombia Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 9.3.3.2. Colombia Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 9.3.4. Chile 9.3.4.1. Chile Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 9.3.4.2. Chile Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 9.3.5. Rest Of South America 9.3.5.1. Rest Of South America Global Preclinical CRO Market Size and Forecast, By Service (2024-2032) 9.3.5.2. Rest Of South America Global Preclinical CRO Market Size and Forecast, By End-User (2024-2032) 10. Company Profile: Key Players 10.1. Envigo Corporation 10.1.1. Company Overview 10.1.2. Business Portfolio 10.1.3. Financial Overview 10.1.4. SWOT Analysis 10.1.5. Strategic Analysis 10.1.6. Recent Developments 10.2. MPI Research 10.3. PRA Health Sciences, Inc. 10.4. Medpace, Inc. 10.5. Pharmaceutical Product Development (PPD), LLC 10.6. PARAXEL International Corporation 10.7. Laboratory Corporation of America, Inc 10.8. Covance Inc. (LabCorp) 10.9. Charles River Laboratories 10.10. Syneos Health 10.11. Thermo Fisher Scientific 10.12. Inotiv, Inc. 10.13. MD Biosciences, Inc 10.14. Eurofins Scientific 10.15. ICON Plc 10.16. Admescope 10.17. Wuxi AppTec 10.18. Crown Bioscience 10.19. Jubilant Biosys 10.20. Novotech 11. Key Findings 12. Industry Recommendations 13. Global Preclinical CRO Market: Research Methodology
